Dillard University offers degrees in the Arts, Business, Humanities, Nursing, STEM, and more. Visit our academic programs today!
Dillard University students become leaders, thinkers, and artists, learning to think critically and act with integrity to make a difference and inspire others to join their mission.
DU COMMUNIVERSITY
Dillard University is the only University in America with a program in African American material culture and the first HBCU to offer a bachelor’s degree in theater Film Studies B.A. Degree.
NEWS

The MHHERC Names New Pre-Health Scholarship Recipients
This year, the Minority Health and Health Equity Research Center

Dillard Wins 3rd Place in HBCU C2 App Design Showcase
Dillard University has achieved an impressive 3rd place in the

Dillard Hosted A Conversation with the New Orleans Black Panthers
Dillard students had a unique opportunity to hear firsthand accounts
Dillard Hosted Under Secretary of State Uzra Zeya
Dillard University had the distinct honor of hosting Under Secretary

Dillard University Concert Choir to Host Annual Spring Concert on April 28
The Dillard University Concert Choir will host its Annual Spring

21 Selected for Dillard University’s Inaugural HEAL Professional Program to Transform Healthcare Advocacy for Breastfeeding Families
The Minority Health and Health Equity Research Center

Rev. Dr. Dominique A. Robinson to Serve as 2024 Baccalaureate Service Speaker
Dillard University is pleased to announce that the esteemed Rev. Dominique A.

Dillard University Advances to 35th Honda Campus All-Star Challenge National Championship Tournament
Dillard University Scholars Compete in 35th HCASC Nationals, vying for

DILLARD UNIVERSITY BECOMES A GROUNDWATER MONITORING SITE
Dillard Joins Deltares’ Disaster Resilience Water Network
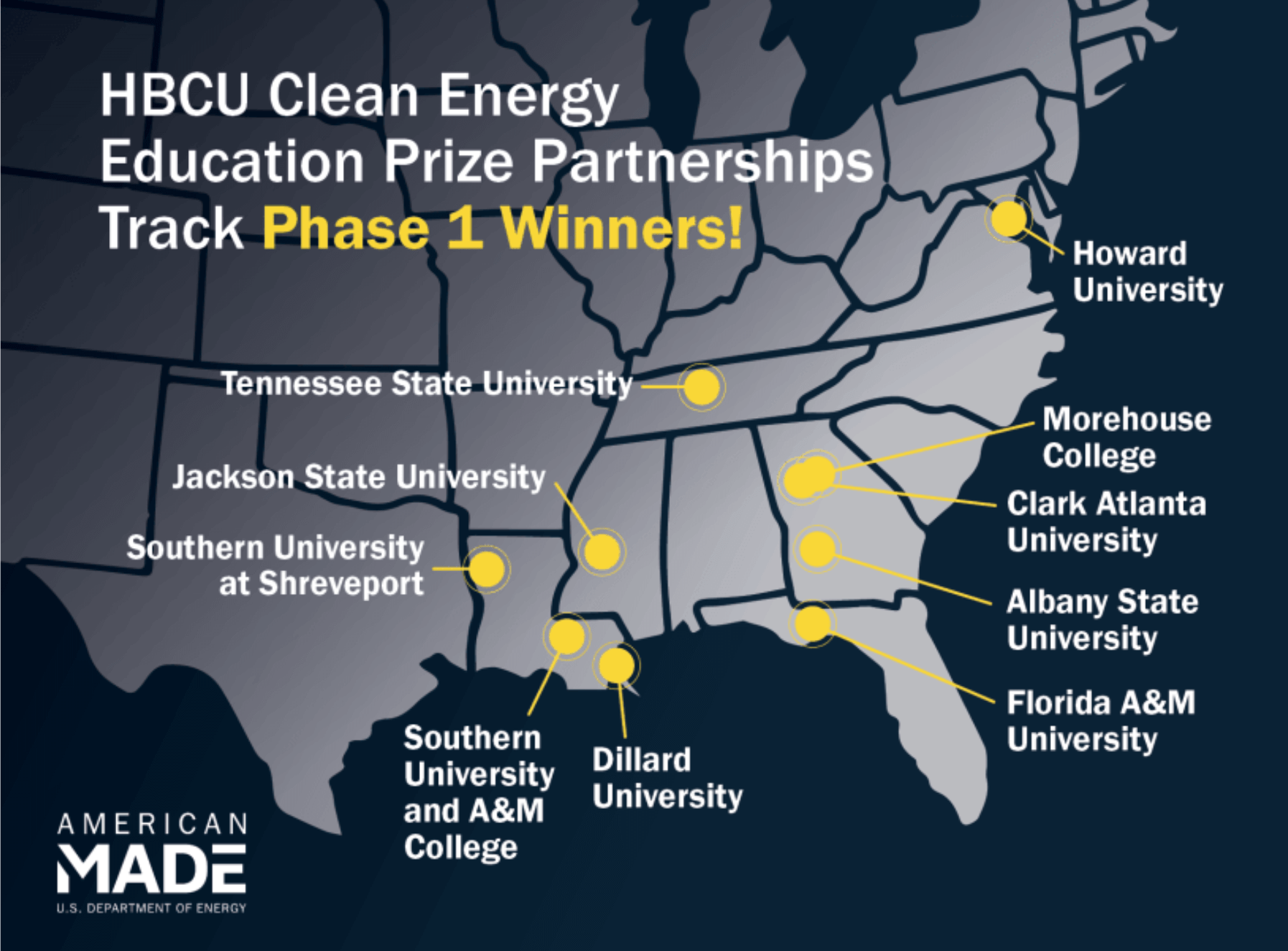
Dillard University Awarded $100K to Support Clean Energy Programming
Dillard Among Inaugural HBCU Clean Energy Prize Winners by U.S.

EVENTS
AT DU
Decision Day

BECOME A BLEU DEVIL!
Experience the legacy, the culture, and the community at Dillard University, Louisiana’s oldest HBCU. Your journey starts here.